Targeted antibiogram for UTIs due to Enterobacteriaceae
in the female population > 12 years old
BEST PRACTICES
Research information note
In 2025, the LabOuest laboratory will systematically apply the HAS recommendations published in October 2023 concerning the management of female urinary tract infections due to Enterobacteriaceae.
Goals
- Guide the practitioner in choosing the antibiotic therapy most appropriate for the situation;
- Preserve our bacterial ecology (prevent antibiotic resistance) by limiting the use of critical antibiotics (notably FQs and carbapenems).
Arbre décisionnel
The table was developed by the French-speaking Society of Infectious Pathology (SPILF) and the French Society of Microbiology (SFM).
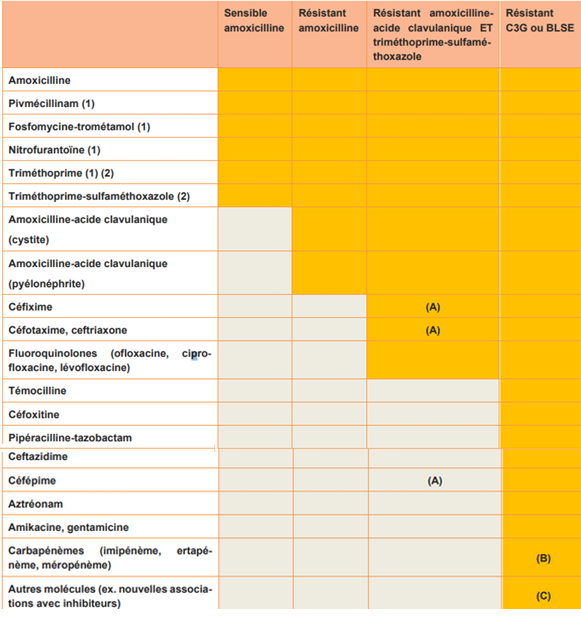
Orange boxes = molecules to be returned
Light gray boxes = molecules not to be returned except resistance(s)
The results report contains at least amoxicillin, pivmecillinam (if Escherichia coli), the combination of fosfomycin/tromethamine, nitrofurantoin, trimethoprim and the combination of trimethoprim/sulfamethoxazole.
If the clinical information is in favor of pyelonephritis, the report specifies at least the sensitivity to amoxicillin as well as to the trimethoprim/sulfamethoxazole combination.
These recommendations do not modify the guidelines for probabilistic antibiotic therapy which take into account the patient’s own risk factors but must guide the practitioner in their therapeutic reassessment at 48-72 hours.
Practical information
The laboratory uses the same diagnostic methods regardless of the population tested. However, the antibiogram results released take these HAS recommendations into account.
In the event of resistance to an antibiotic of therapeutic interest, this information is systematically reported in the results report.
The complete antibiogram remains available upon request from the clinician.