Cervical cancer screening (HPV test)
In France, high-risk human papillomavirus (HR-HPV) are each accountable for some 3,000 newly detected cases of invasive cervical cancer and over 1,000 deaths. This is one of the only forms of cancer for which the prognosis is worsening.
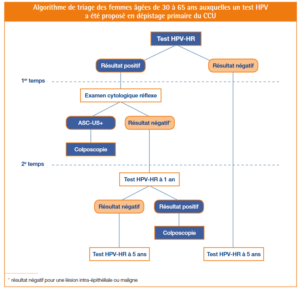
Following recommendations of the French Health Authority (HAS) in July 2019, the decree published on 25th March 2020 and applicable from 1st April 2020 both amended and widened the conditions for reimbursement of HPV screening as part of the detection process for cervical cancer, notably by recommending a primary HR-HPV test for women between the ages of 30 and 65 years old.
A significant number of women avoid classic gynaecological supervision with a Pap test for many reasons due to acceptability and practicality. It is in this framework that the French Health Authority recommended the use of self-sampling swabs.
A number of studies have shown that the performance of these swabs is identical to a self-sampled HPV swab or even a swab undertaken by a healthcare professional. This HR-HPV swab test is paid for by French Social Security funding and should be explicitly prescribed in lieu of a histopathological examination of which the diagnostic sensitivity is significantly lower and of which the subjective interpretation is operator-dependent.
- Self-swabs can be conducted in the patient’s home or in a laboratory. The self-swab kit is very easy to use and comprises a dry swab and nucleic acid storage container. The swab remains stable for 72 hours at room temperature.
If the result is positive, the patient is referred for a cervical smear test by a doctor, with a new endo-cervical smear test.
-
- If a cervical smear test is undertaken directly in a medical centre with an appropriate swab kit (kits provided by LabOuest on request),
with a prescription including a clear indication for HR-HPV screening, the swab sample will be sent for anatomopathological analysis for all positive HPV tests, directly from the laboratory.
- If a cervical smear test is undertaken directly in a medical centre with an appropriate swab kit (kits provided by LabOuest on request),
with a prescription including a clear indication for HR-HPV screening, the swab sample will be sent for anatomopathological analysis for all positive HPV tests, directly from the laboratory.
LabOuest offers a new molecular biological technique:
-
-
- Multiplex real-time PCR, Seegene Anyplex II HR-HPV, EC-IVD labelled and approved by the CNR.
- which is able to simultaneously detect 14 high-risk human papillomavirus (HR-HPV) genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68
-
We undertake one round of testing per week. The frequency of testing will gradually increase in proportion with the number of swabs to be tested.
ALL ABOUT HPV
Epidemiology
-
-
- Small double-strand highly resistant DNA viruses;
- Infection following direct contact with the squamous-epithelium on the skin and mucosa, most frequently during sexual intercourse. HPV is one of the most frequent sexually-transmitted infections throughout the world;
- 80% of women will be infected by an HPV during their lifetime;
- In the vast majority of cases, these infections are asymptomatic and are rapidly undetectable in bodily tissues. Around 90% of infections are no longer detectable after 2 years (immune system clearance);
- In women under 30 years old, the prevalence of transitory HPV infections is relatively high due to a period of higher sexual activity before then regressing after 30 years old;
- 3/4 of cases are diagnosed in women aged between 25 and 64 years old;
- There is an insufficient rate of testing coverage (~60%).
-
Classification
-
-
- Over 200 genotypes
- 3 categories: :
- high risk cancer-causing HPV (HR-HPV): 16 and 18 are the most frequent
- possibly cancerous HPV
- non-cancer-causing HPV (6 and 11) responsible for warts
-
Cervical cancer
-
-
- All forms of cervical cancer are linked to a persistent cancer-causing papillomavirus infection
- in France, HR-HPV are responsible for 3,000 new cases of cervical cancer each year
- 60% of cervical cancer are due to HPV-16 and 15% due to HPV-18
-
Diagnosis
-
-
- HR-HPV test: cervical swab or self-test
- women between 25 and 30 years old: primary screening uses a cytological examination (cervical swab)
- women between 30 and 65 years old: primary screening uses a molecular biological method (HR-HPV test by PCR)
-